Close Menu
© 2024 Dr. Paul Lohmann GmbH & Co. KGaA
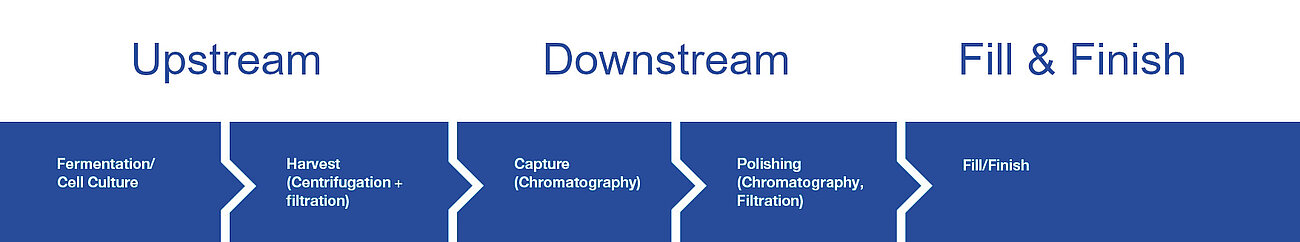
Our Salts are used in all biopharmaceutical production streams - upstream, downstream, fill & finish - in the highest purity and quality. They are an indispensable part of bioproduction and are used as nutrients, processing aids or excipients in the final formulation.
In order to meet the particularly high requirements of this industry, Dr. Paul Lohmann® has created its own DPL-BioPharm standard.

The demanding requirements of the biopharmaceutical industry in terms of quality, purity and efficacy require profound approaches to ensure the integrity of raw materials. This includes the entire supply chain as well as the subsequent production processes.
Therefore, we have established DPL-BioPharm Packaging, which meets the highest quality and safety standards. We guarantee a fiber-free double packaging under GMP to prevent contamination as well as powder caking:
We also realize customized packaging’s according to your needs.

Calcium
Iron
Magnesium
Aluminium
Copper
Manganese
Potassium
Sodium
Sample Request
Product Finder
Contact